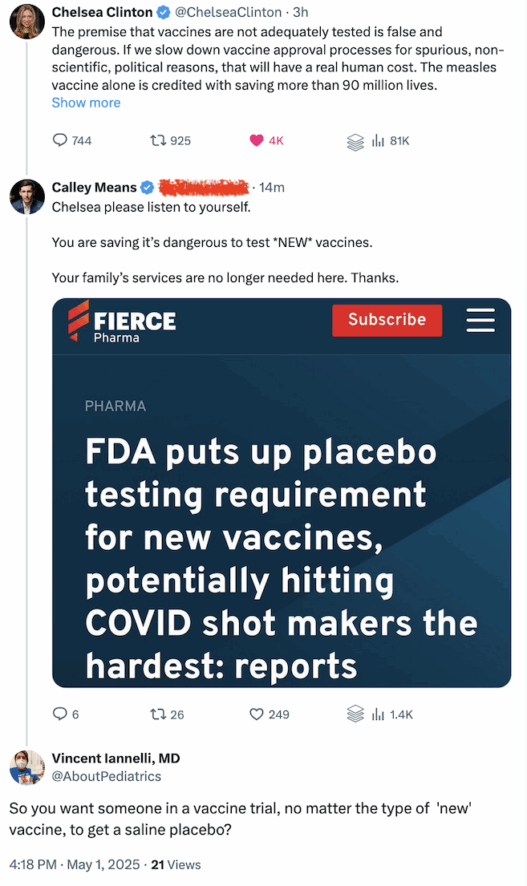
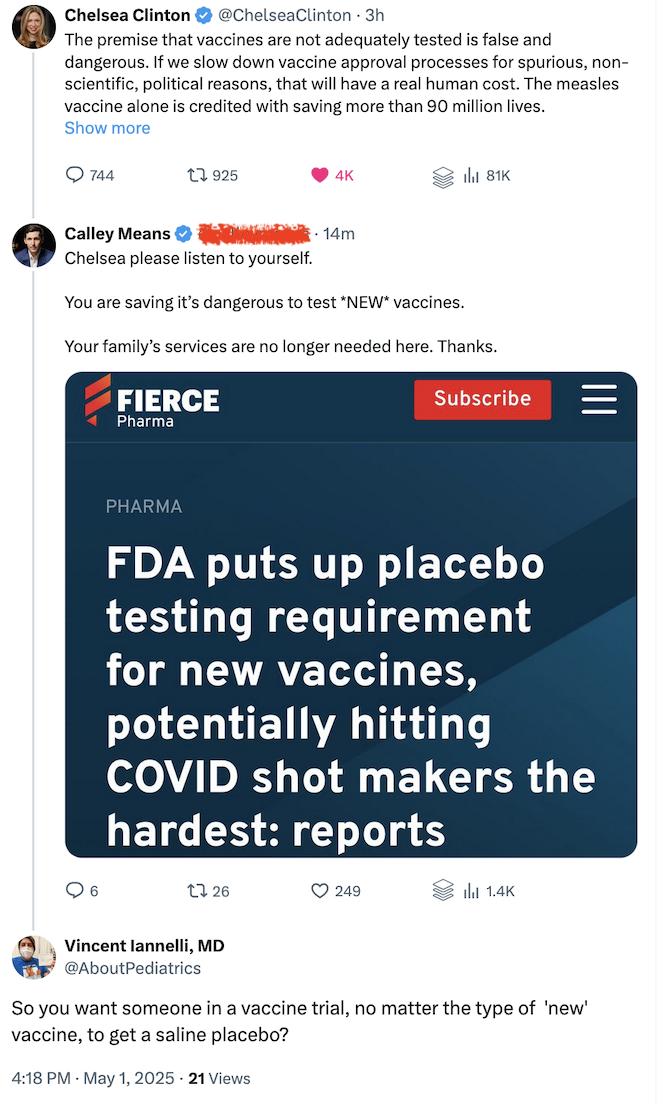
On the surface, I’m sure it sounds like a good idea to test all new vaccines with a saline placebo.

After all, why wouldn’t you want all new vaccines to be tested that way?
Should All New Vaccines Require Testing With a Saline Placebo?
Well, for one thing, it is quite unethical!
“Placebo use in vaccine trials is clearly acceptable when (a) no efficacious and safe vaccine exists and (b) the vaccine under consideration is intended to benefit the population in which the vaccine is to be tested.”
Placebo use in vaccine trials: Recommendations of a WHO expert panel
You see, folks in vaccine trials should get a benefit from being in the trial.
With some vaccines, the benefit is that a disease in their community will be conquered.
“Placebo use in vaccine trials is clearly acceptable when (a) no efficacious and safe vaccine exists and (b) the vaccine under consideration is intended to benefit the population in which the vaccine is to be tested. In this situation, a placebo-controlled trial addresses the locally relevant question regarding the extent to which the new vaccine is better than nothing, and participants in the placebo arm of the trial are not deprived of the clinical benefits of an existing efficacious vaccine.”
Placebo use in vaccine trials: Recommendations of a WHO expert panel
And that’s a big benefit!
That’s how you can get away with using a saline placebo in some new vaccine trials, like for HIV, malaria, or even COVID.
“However, randomised, placebo-controlled trial designs often raise ethical concerns when participants in the control arm are deprived of an existing vaccine. Furthermore, testing a new vaccine against placebo is scientifically and ethically fraught when the hypothesis being tested is whether an experimental vaccine is more efficacious than one already in use in the same or in other settings.”
Placebo use in vaccine trials: Recommendations of a WHO expert panel
But if there is already another type of vaccine for the disease you are targeting, it is unconsidered unethical to give folks in vaccine trials a saline placebo.
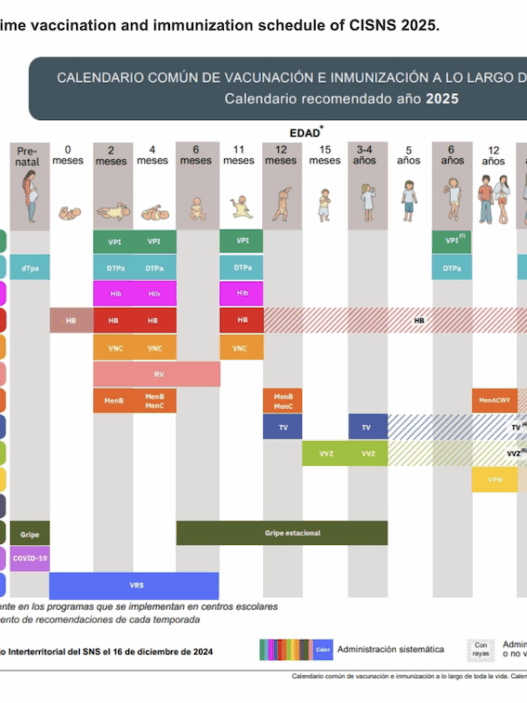
You instead give another type of vaccine that targets that same or a similar disease, whether it is pneumonia or meningitis, or hepatitis, etc. Or a newer version of the vaccine that has already been safety tested, that way they aren’t left unprotected.
How does that work?
It works very well!
Placebos do not have to simply be saline!
“Placebo Control – A comparator in a vaccine trial that does not include the antigen under study. In studies of monovalent vaccines this may be an inert placebo (e.g. saline solution or the vehicle of the vaccine), or an antigenically different vaccine. In combined vaccines, this may be a control arm in which the component of the vaccine being studied is lacking.”
WHO on the Guidelines on clinical evaluation of vaccines: regulatory expectations
The dangerous idea that saline placebos are always necessary was created by anti-vaccine influencers to make people think that vaccines have never been safely tested.

Dangerous ideas that will lead to fewer life-saving vaccines being approved.
“It’s just not correct. They obviously don’t understand how vaccines are approved and how one obtains safety data.”
Michael Osterholm to WaPo
Dangerous ideas that are becoming policy now that RFK Jr is Secretary of HHS…
More on Saline Placebos
Last Updated on May 1, 2025